FOLLISTIM® AQ Cartridge Instructions for Use
Watch the video to learn about self-injection with the FOLLISTIM Pen®
Scroll for More Info
Instructional Quick Guide for FOLLISTIM® AQ Cartridge (follitropin beta injection)
Directions provided here are abbreviated. They should be used only after receiving complete instructions from your health care professional.
To give yourself the injection, you will need:
A clean, dry surface, alcohol and cotton balls, or alcohol pads, sterile gauze, anti-bacterial soap, and a puncture-proof container to discard used supplies.
FOLLISTIM Pen®

A FOLLISTIM AQ Cartridge

A new BD Micro-Fine™ Needle


Prepare your FOLLISTIM Pen
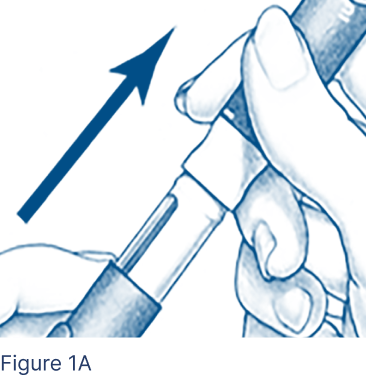

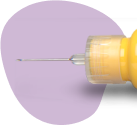
Pull the protective cap off the Pen Body (Figure 1A) and put it aside on a clean, dry surface.
- Unscrew the entire Pen Body from the Cartridge Holder.
- Place Pen Body and Cartridage Holder on a clean, dry surface.
- Remove the FOLLISTIM AQ Cartridge from its package and clean the rubber inlay of the Cartridge with an alcohol pad.
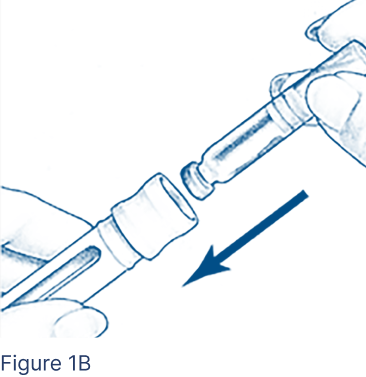

Insert the Cartridge into the Cartridge Holder (Figure 1B), metal rimmed cap end first.
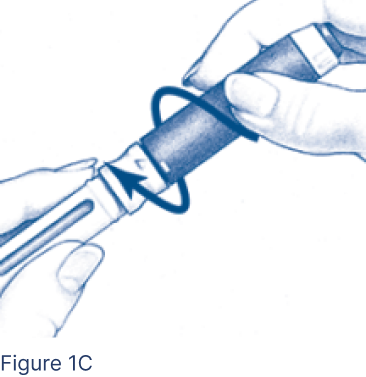

Screw Pen Body fully onto the Cartridge Holder containing the inserted Cartridge (Figure 1C), making sure there are no gaps.
- Line up the arrow with the middle of the yellow alignment mark.
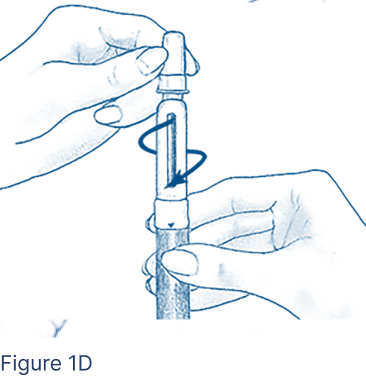

Clean the open end of the Cartridge Holder with an alcohol pad. Peel off the protective paper seal from the BD Micro-Fine Pen Needle that is in its Outer Needle Shield. Attach a new BD Micro-Fine Pen Needle after you make sure there is a FOLLISTIM AQ Cartridge in the FOLLISTIM Pen. Push the end of the Cartridge Holder into the Outer Needle Shield. Screw tightly together (Figure 1D).
Your FOLLISTIM Pen is now assembled and loaded.
Never touch the needle and never place an open needle on any surface.
You must use a new BD Micro-Fine Pen Needle with each injection. Use an alcohol pad to clean about a 2-inch area of skin where the needle will enter.
Set your dose
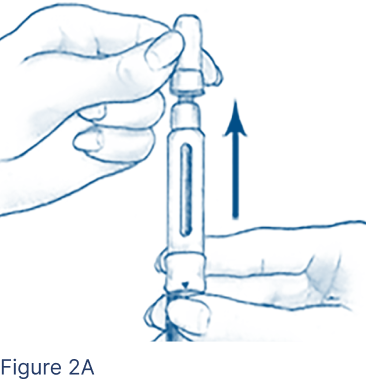

Gently pull off the Outer Needle Shield, leaving the Inner Needle Shield in place (Figure 2A), and set aside.
- Use an alcohol pad to clean about a 2-inch area of skin where the needle will enter.
- Do not discard the Outer Needle Shield.
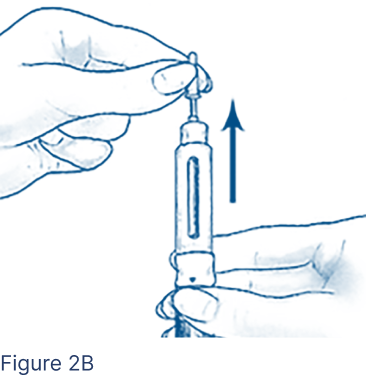

Carefully remove the Inner Needle Shield (Figure 2B) and discard.
- Do not touch the needle or let it touch any surface while uncapped.
- Hold the FOLLISTIM Pen® with the needle pointing upwards. Tap the cartridge holder gently with your finger to force air bubbles to the top of the needle.

Dial the Dosage Knob until you hear one click. With the needle pointing upwards, push in the Injection Button.
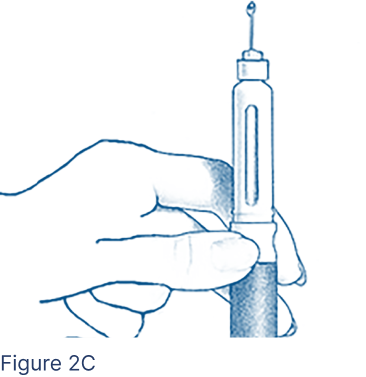
Look for a droplet of medicine at the needle tip (Figure 2C). If you do not see a droplet, repeat steps above; otherwise you might not receive the correct dose.
If you have already used the FOLLISTIM® AQ Cartridge (follitropin beta injection) and you need to give yourself another dose, attach a new BD Micro-Fine™ Pen Needle and look for a droplet forming at the tip of the needle. If you do not see a droplet, repeat the previous 2 steps.
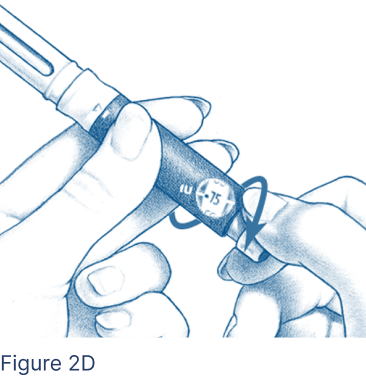

For doses of 50 International Units (IU) up to 450 IU, turn the Dosage Knob until the correct dosage aligns with the dosage markers on each side of the Dosage Window (Figure 2D).
If you dial past your dose, do not turn the Dosage Knob backward
(you will not damage the Pen, but medicine will be lost). Instead, continue turning until you pass the 450 IU mark, as far as it will turn. The Dosage Knob must move freely. Push the Injection Button in as far as it will go. Start dialing from “0” upwards until you reach the correct dosage.
Never try to correct a dose-dialing mistake while the needle is in your skin. This may result in an incorrect dose. If your prescribed dose exceeds the deliverable dose of the FOLLISTIM Pen or exceeds the amount remaining in the Cartridge, you will need to give yourself more than one injection.
You are now ready to complete your injection.
The best places to inject are just below your belly button or the upper outer area of your thigh.
Inject
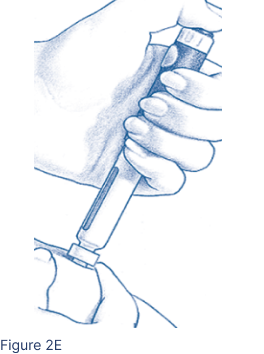
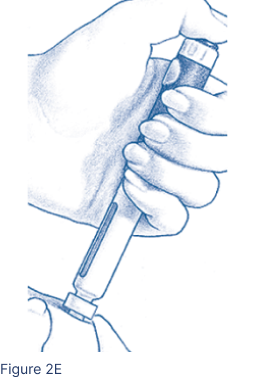
Pinch the cleansed skin area between 2 fingers. With your other hand, insert the entire Pen Needle straight into the skin at a 90-degree angle (Figure 2E). Press the Injection Button all the way. Wait 5 seconds. The middle of the Dosage Window should display a dot next to the “0”. Pull the needle out quickly and apply pressure to the injection site with an alcohol pad.
If the number in the Dosage Window does not read “0” and you cannot push the Injection Button all the way in, do not try to force down the button. Your FOLLISTIM AQ Cartridge is probably empty. This means you have not received your full dose. Do not adjust the setting on the Dosage Scale. The number in the Dosage Window is the amount needed to complete your prescribed dose. Write down this number. Remove the needle and dispose of it properly. Reset the Dial Window to “0” by turning the Dosage Knob past the 450 IU mark as far as it will turn and pushing the injection button all the way in. Repeat instructions from “Ready (►) Prepare Your FOLLISTIM Pen to load a new Cartridge, attach a new needle and inject the remaining drug to complete your dose”.
Storage and Disposal
Each Pen Needle is for one injection only. Immediately following injection, remove and dispose of the used Pen Needle. The Pen Needle must be removed from the FOLLISTIM Pen prior to storage.

Storing the FOLLISTIM AQ Cartridge
Store refrigerated 2°-8°C (36°-46°F) until dispensed. Upon dispensing, the product may be stored by the patient at 2°-8°C (36°-46°F) until the expiration date, at or below 25°C (77°F) for 3 months or until expiration date, whichever occurs first. Once the rubber inlay of the FOLLISTIM AQ Cartridge has been pierced by a needle, the product can only be stored for a maximum of 28 days at 2°-25°C (36°-77°F). Keep away from light. Do not freeze.
Patient support from Organon and ReUnite Rx
Support is available for eligible self-paying patients. Organon continues to collaborate with ReUnite Rx to assist eligible cash-paying fertility patients. If you need additional support for out-of-pocket costs for an Organon fertility medication, there may be other programs that can help.